Medical
About Us
Products
Fully automated faecal test analyser
OC-SENSOR Ceres
OC-SENSOR Ceres is the faecal test analyser with simple to use and compact design for faecal immunochemical test and faecal calprotectin test. OC-SENSOR Ceres encompasses all the technological advances with comparable analytical performance to the high-end model.

Faecal Immunochemical Test (FIT) and Faecal Calprotectin (FCa) are non-invasive tests that aid in the detection of serious bowel disease in symptomatic patients.
Due to the limited endoscopic resources and access to healthcare systems, FIT and FCa have become valuable markers for informed decision making in the clinical pathway. This enables patients at highest risk to be identified swiftly and reduce the number of unnecessary colonoscopies.

Sampling Bottle is common for FIT, FCa and more test parameters.
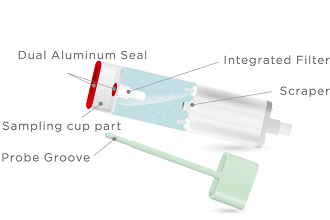
Sample preparation is completed in a single stool collection.
By patients bringing samples collected at home, faecal testing within an outpatient setting allows a decision to be made for referral or further investigation following a single consultation by General Practitioner (GP).

Operation is simple: just load the samples and press the start button.
Healthcare professionals can easily perform quantitative faecal test, even if they are not familiar with analyser operation.

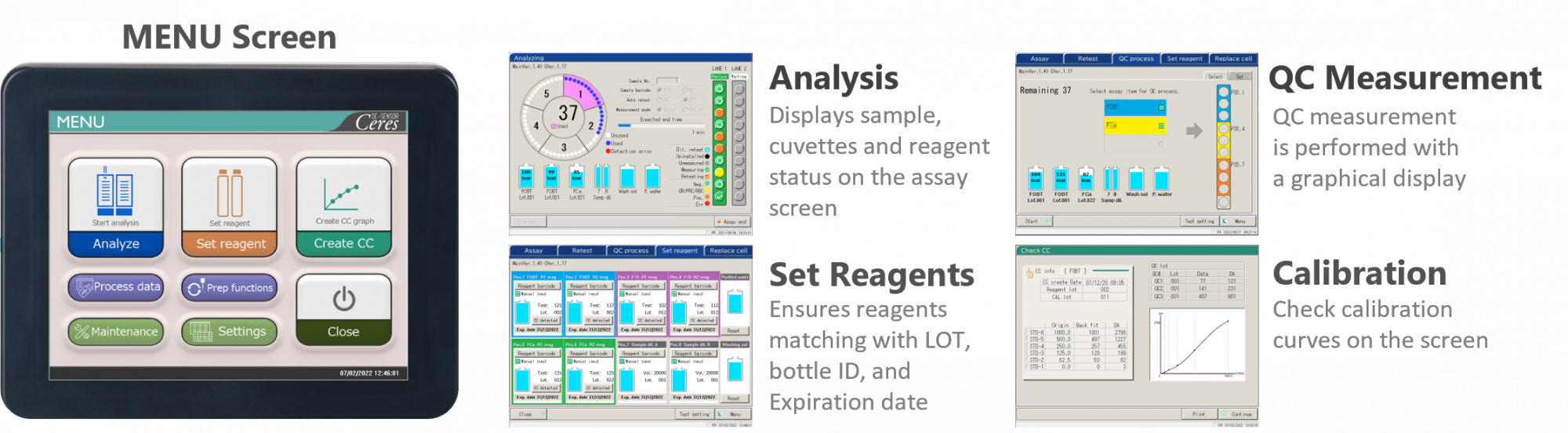
The simplicity known from the previous compact model of OC-SENSOR stays with OC-SENSOR Ceres. The new OC-SENSOR Ceres has an improved operation screen design and highly sensitive touch sensor, making it simpler and easier to use.
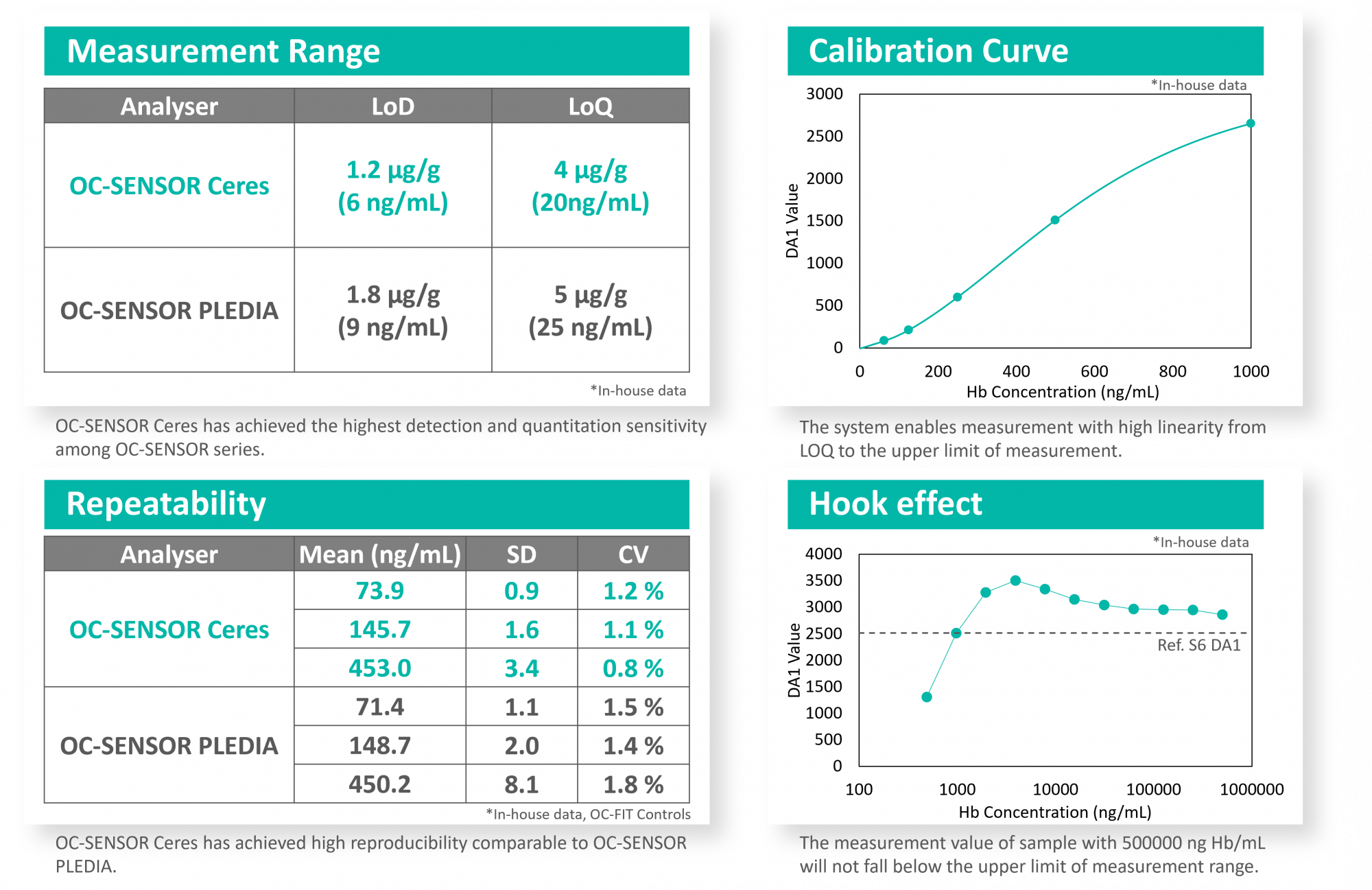
OC-SENSOR Ceres achieves the analytical performance comparable to the high-end model, OC-SENSOR PLEDIA, in a local laboratory environment.
*Examples of measurement with FIT are shown below. Measurements and calculations were performed based on Clinical Laboratory Standard Institute (CLSI) guidelines.

OC SENSOR Ceres has introduced a build-in refrigerator. There is no need to store back the reagents in a refrigerator after use. Reagents are stable for 4 weeks on-board, which is a sustainable option for those laboratories with a small number of samples.
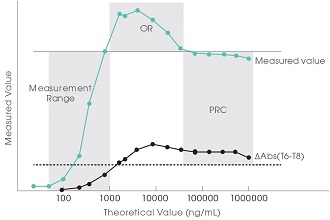
If the ΔAbs of the photometric point in the initial reaction phase is above the lower limit of the PRC and the measured value is below the upper limit of measurement, the sample is recognized as within the prozone (PRC region). Prozone of highly concentrated sample is checked through the Primary Rate Check (PRC) method.
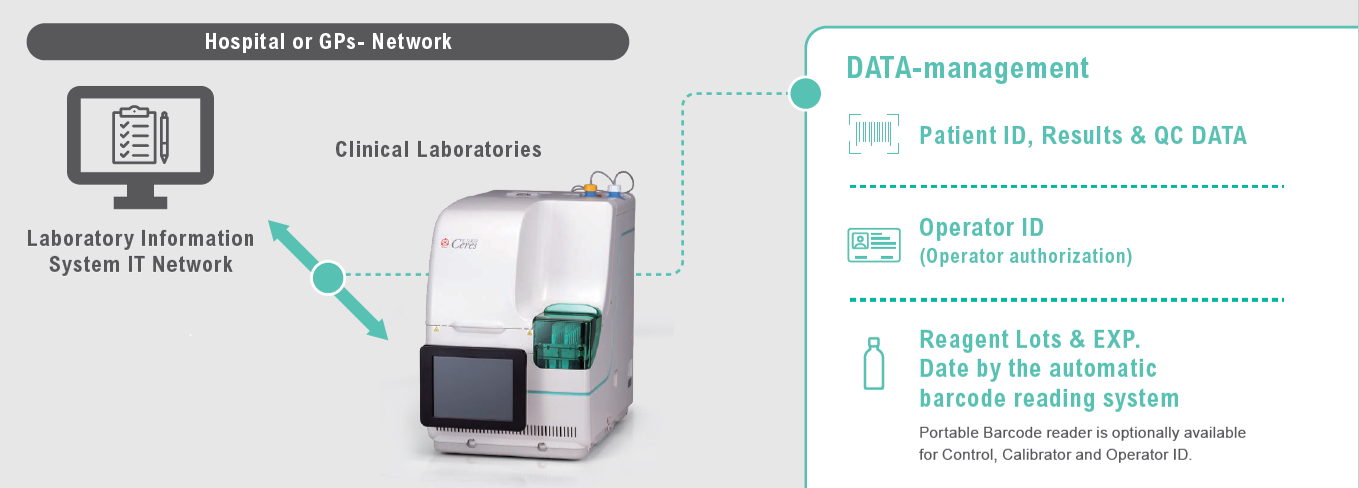
OC-SENSOR Ceres is equipped with functions comparable to high-end models. In addition to test order and results output via LIMS bidirectional connection, QC results can be displayed in X-R control chart. Managing reagent lots and expiration dates by barcode makes it easier to conform to ISO 15189 standards.

Enhanced Barcode System
The information is automatically read by the built-in barcode reader; Patient ID for Sampling Bottles, lot number, expiry date and test item for reagents.
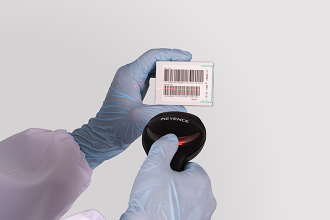
Portable barcode reader is optionally available for:
・Operator ID
・Calibrator lot number, expiry date, test item
・QC lot number, expiry date, test item and reference range
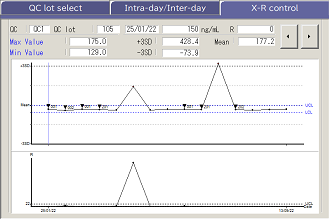
QC Measurement & Monitoring
Simultaneous Measurement of up to 3 different QC levels are possible. Management for instrument and reagent conditions are monitored through the X-R QC chart, auto CV calculation and a host of other functions.
OC-SENSOR Ceres supports bi-directional interface for real-time data reporting to Laboratory Information Management System (LIMS).
|
Principle |
Latex agglutination immunoturbidity |
|
Test items |
Faecal Immunochemical Test, Faecal Calprotectin |
|
Throughput |
Up to 90 tests / hour |
|
Sample run capacity |
10 samples x 2 racks, Continuous loading |
|
First result |
FIT: 8 min, FCa: 10min, FIT and FCa: 11min |
|
Dimensions |
W 360 mm x D 625 mm x H 545 mm |
|
Miki I, Maki H, Ikuhiro M, Yoh H. Evaluation of the analytical performance of the new compact, tabletop, discrete-type automated clinical chemistry analyser "OC-SENSOR Ceres" for fecal occult blood testing. The Journal of Clinical Laboratory Instruments and Reagents. 2021; 44(3):258-264. “We evaluated the analytical performance of OC-SENSOR Ceres in fecal occult blood quantification and obtained overall good results." |
To learn more about OC-SENSOR reagents performance, please refer to our publication list.