Medical
About Us


Our Fields
Eiken Chemical launched sales of fully automated FIT analyser in 1989. Since then, we have been developing automated FIT system “OC-SENSOR” for 30 years worldwide. Approximately 100 million tests are performed per year in more than 45 countries. OC-SENSOR has been used in many academic publications and national guidelines [*3, and see publication page].
In some countries, national guidelines recommend faecal immunochemical test (FIT) as a method of population based screening for colorectal cancer [*1 European Commission, *2 UK, *3,4 US, *5 Canada]. In the recommendation statement of U.S. Preventive Service Task Force, our product OC-Sensor is mentioned as “the best test performance characteristics (ie, highest sensitivity and specificity) [*3].”
Watch the video to learn about our experiences with FIT screening.
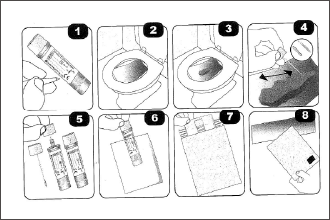
Collection Paper
Stool sample collection paper: To take stool sample easily and adequately, placing a Collection paper can help catch stool in the toilet bowl. Eiken Chemical provides flushable paper that sampling instruction is printed.
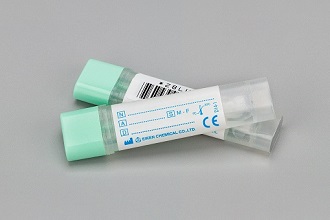
OC-Auto Sampling Bottle 3
Stool sampling devices: OC-Auto Sampling Bottle 3 is a palm-sized stool collection device. Patients scrape the surface of stool with the probe and insert it into the bottle. After closing the device properly, the liquid does not leak out easily.
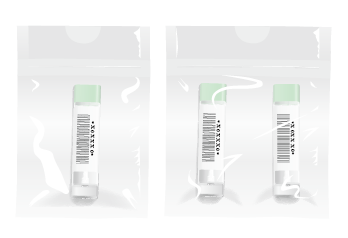
Special Envelope (left: for 1 day, right: for 2 days)
Envelope for mailing: We developed an envelope integrating absorbent pat, called Special Envelope, for OC-Auto Sampling Bottle 3. Two types of envelope are available; for 1-day and 2-day sampling method.
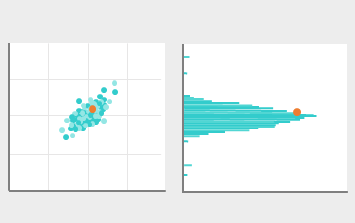
Data example
Eiken Quality Control Survey (EQCS):
We offer an external quality assessment service (Eiken Quality Control Survey; EQCS) for OC-SENSOR users through our local distributors. The service started in 1995 and currently more than 1500 laboratories from 20+ countries participate in the program every year. EQCS supports laboratories to assess their performance objectively by comparison of the testing results among the participants. *Currently we are conducting surveys for FIT only.
Cutoff value adjusting: Users can choose cutoff to maximize sensitivity and specificity. Studies were conducted to suggest the appropriate values in population screening [*7].
2 sized analysers: We can offer two different sized fully automated analysers OC-SENSOR PLEDIA and OC-SENSOR Ceres which can be chosen according to the scale of screening.