Medical
About Us


Our Fields
The fecal immunochemical test (FIT) is widely used as an approach for non-invasive screening for colorectal cancer all over the world. See how OC-SENSOR FIT is being adopted.
Colorectal cancer is the third most common cancer and the second cancer-related death worldwide. It is estimated 1.8 million new cases are diagnosed and about 880,800 deaths from colorectal cancer occur in 2018. The incidence and mortality rate are relatively high in Europe, North America, Oceania and East Asia [*1].
Major symptoms can include: persistent change in bowel habits, blood in/on stool except hemorrhoids, abdominal pain, rectal mass, appetite loss and unexpected weight loss [*2].
Cancer may be already progressing when you notice the symptoms.
At the same time, however, the survival rates of colorectal cancer is high when diagnosed at the earlier stage. According to the report from National Cancer Intelligence Network in 2009 [*3], 5-year relative survival rate of patients in England is 93.2% at the earliest stage of colorectal cancer (Dukes A) compared with only 6.6% of survival rate when the cancer spread to other parts of the body (Dukes D).
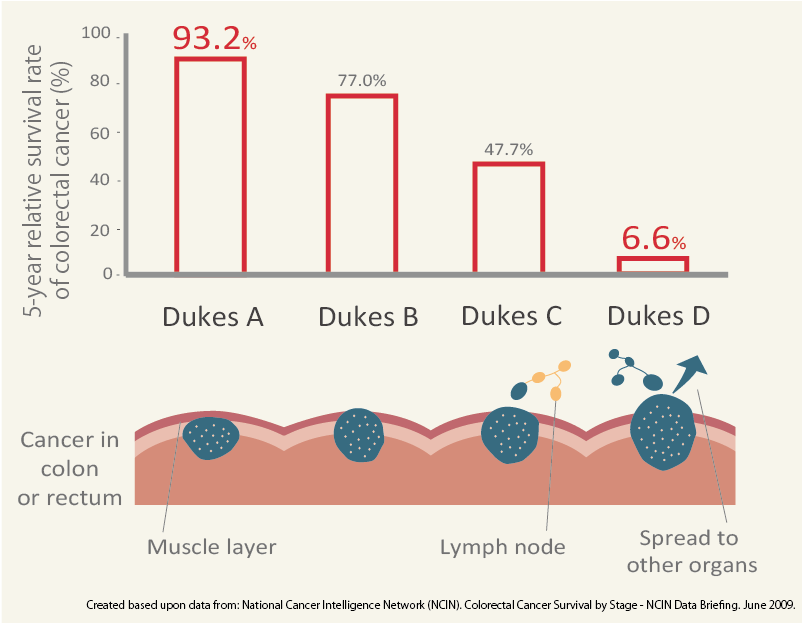
Created based upon data from: National Cancer Intelligence Network (NCIN). Colorectal Cancer Survival by Stage - NCIN Data Briefing. June 2009. http://www.ncin.org.uk/publications/data_briefings/colorectal_cancer_survival_by_stage
Fecal occult blood test is often used for large population screening for colorectal cancer. People over designated age (e.g. 50 years old) are invited to take the program. The stool sample collections are performed simply by patients, and those who get positive results are normally informed to refer colonoscopy examination operated by doctors.