Medical
About Us
Products
 National Guidelines
National Guidelines|
U.S. Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965-1977. PubMed > |
|
Halloran S, Launoy G, Zappa M. European Commission. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition – Faecal Occult Blood Testing. Endoscopy 2012; 44:SE65–SE87. PubMed > Publicaions Office of EU > |
 OC-SENSOR study
OC-SENSOR study|
Young GP, Symonds EL, Allison JE, et al. Advances in Fecal Occult Blood Tests: The FIT Revolution. Dig Dis Sci (2015) 60:609–622. PubMed > |
|
Allison JE, Fraser CG, Halloran SP, Young GP. Population screening for colorectal cancer means getting FIT: the past, present, and future of colorectal cancer screening using the fecal immunochemical test for hemoglobin (FIT). Gut Liver. 2014;8(2):117–130. PubMed > |
|
van Rossum LG, van Rijn AF, Laheij RJ, et al. Cutoff value determines the performance of a semi-quantitative immunochemical faecal occult blood test in a colorectal cancer screening programme. Br J Cancer. 2009;101(8):1274-81. PubMed > |
|
O'Driscoll S, Piggott C, Benton SC. Evaluation of a faecal calprotectin method using the OC-SENSOR PLEDIA. Clin Chem Lab Med. 2022 Mar 14;60(6):901-906. PubMed > |
|
Butenas J, Ayling RM. Clinical evaluation of the OC-Sensor Pledia calprotectin assay. Clin Chem Lab Med. 2022 Sep 12;60(11):1780-1785. PubMed > |
We have 3 choices for your capacity of laboratory.
| Laboratory scale | Quantitative method | Qualitative method |
|---|---|---|

Large laboratory |
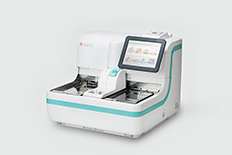
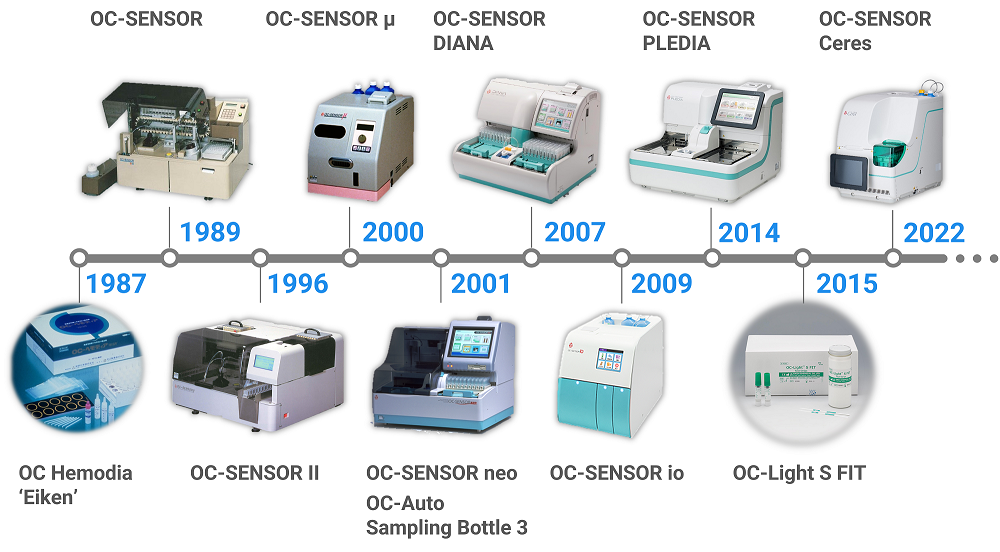
OC-SENSOR PLEDIA High-end faecal test analyzer for Faecal immunochemical test and Faecal calprotectin test. The throughput is 320 tests/hour. more |
|

Small laboratory |

OC-SENSOR Ceres Accessible, compact, and simple to use design with comparable analytical performance to the high-end model. The throughput is 90 tests/hour. more |
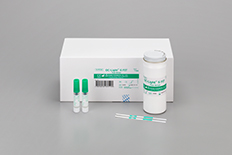
OC-Light™ S Manual test strip for faecal immunochemical test with qualitative results. more |