Medical
About Us


Our Fields
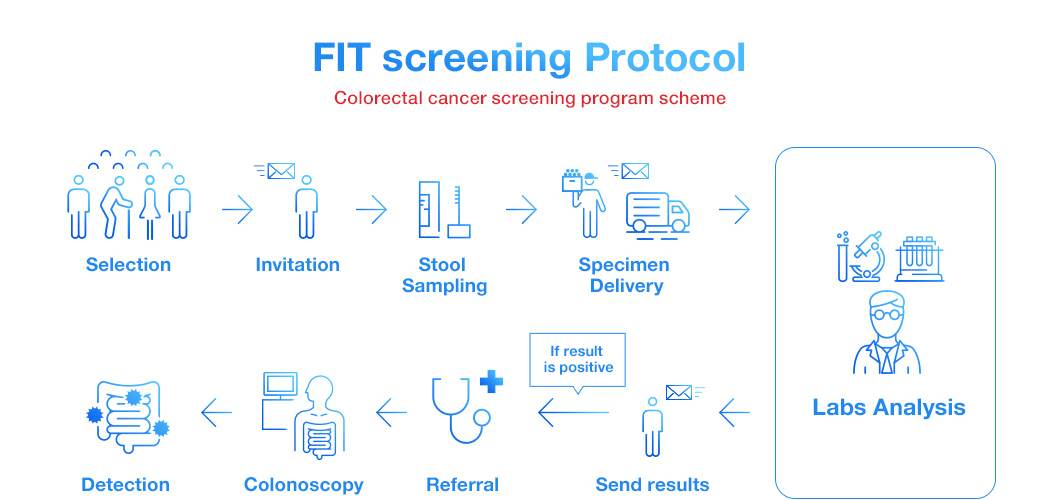
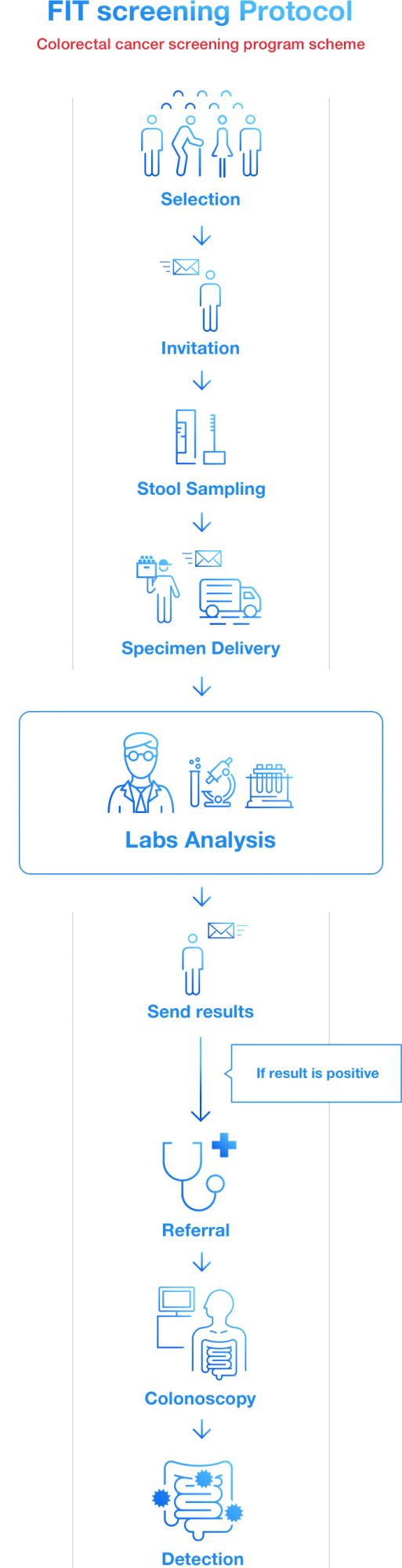
Fecal immunochemical test (FIT) is used for detecting invisible hemoglobin in red blood cells on the surface of stool. The principle of FIT is antigen-antibody reaction which has specificity to human hemoglobin in lower digestive tract. When the test result shows significant level of hemoglobin, it may be a sign of bleeding caused from gastrointestinal diseases.
Watch the video to see how OC-SENSOR FIT is being adopted for colorectal cancer screening.
Some countries offer FIT as national screening program of colorectal cancer. Each government or organization invites people within eligible age (e.g. over 50 years old) to take the test. Patients take fecal samples privately at home and send them to GPs or laboratories, then the test results are informed to them after certain period. If patients get an abnormal result, however, it does not necessarily mean they have colorectal cancer. They are recommended to take a colonoscopy examination as a referral.
When hemoglobin is found on the surface of stool, it is possibly caused from blooding in digestive tract.
Fecal occult blood test (FOBT) has been conducted in two methods; chemical method called guaiac test or gFOBT, and immunoassay called fecal immunochemical method (FIT) or iFOBT.
Guaiac test (gFOBT) is developed as a method of detecting hemoglobin on stool with peroxidase activity which changes the color of test paper by oxidation. Studies had been conducted to use the method for colorectal cancer screening and the results showed guaiac test decreases the incidence [*1] and the mortality [*2].
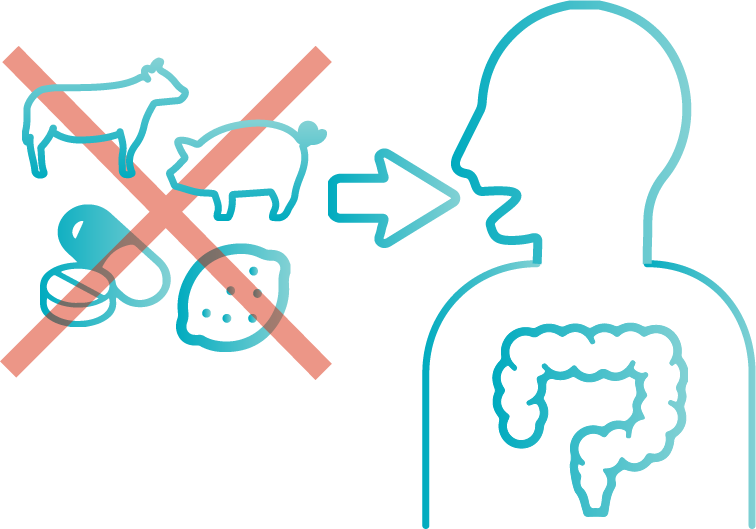
However, guaiac test has uncertainty for the result because some factors affect it. One reason is peroxidase activity will be affected by diet and drug, for example, eating certain amount of red meat, high peroxidase-containing fruits and vegetables, vitamin C supplements, or having medicines containing aspirin [*3]. These Patients should avoid them before taking the test, or it can cause false positive or false negative results [*3].
Fecal immunochemical test (FIT) is another way to detect hemoglobin on the surface of stool. The principle is antigen-antibody reaction which is specific to human hemoglobin from lower digestive tract. There are no drug and dietary restrictions for patients. Moreover, the participation and detection rate of FIT is higher than guaiac test in population screening [*4].
Also, FIT enables quantitative measurement of hemoglobin on automated analyzers. Each test center can adopt different cutoff concentration to set an appropriate positivity rate, which helps minimize the number of colonoscopy needed for referral depending on the resource. In 2010, European Commission reported FIT as an “both analytically and clinically more sensitive and specific [*5].”

FIT can be also used in symptomatic patients who are at “low but not no risk” of having colorectal cancer. OC-SENSOR is endorsed as the cost-effective FIT to assess the risk of colorectal cancer and to guide further investigation in National Institute of Health and Care Excellence (NICE DG30) guidelines [*6].