Medical
About Us


Our Fields
FTSE FAHMS FGESA AGAF, MD FRACP
Matthew Flinders Distinguished Emeritus Professor, Flinders University

Graeme Young graduated MB, BS in 1969 from the University of Melbourne. He studied at Washington University, St Louis in 1978-1980 after which he obtained his MD by research thesis in 1981. Following academic appointments at University of Melbourne, in 1997 he was appointed as the foundation Professor of Gastroenterology at Flinders University of South Australia. In 2011 he was appointed Professor of Global Gastrointestinal Health at Flinders University and was honoured with the title Matthew Flinders Distinguished Professor. He is a Fellow of two academies and three professional bodies.
Key awards include: 2017: Winner of the Australian Museum Eureka Prize for Innovation in Medical Research. 2014: Membership of the Order of Australia (AM). 2013: South Australian Scientist of the Year. 2009: Distinguished Research Prize of the Gastroenterological Society of Australia.
He was a founding member of the World Endoscopy Organization’s (WEO) Colorectal Cancer Screening Committee in 1998. He chaired this committee in 2005-2013 and remains an active member, leader of major international publications and health policy in the field. During the first 20 years of the committee, the number of countries implementing population-based organised colorectal cancer screening rose from just 2 to more than 40, most of which screen using faecal immunochemical tests for haemoglobin (FIT).
He is an internationally recognised expert on colorectal cancer (CRC) screening and biomarkers, screening policy and its implementation. His early work with international colleagues established the key role that FIT would play in establishing CRC screening in many countries. Graeme has published nearly 500 scientific and medical papers, with over 400 colleagues –several hundred of these are international colleagues.
CRC is a significant cause of cancer-related deaths and thus a major health problem. It is ranked in the top ten diseases for health burden by the World Bank [A]. There is wide geographic variation in CRC incidence [B], being higher in developed than developing countries. Incidence is increasing in those with growing affluence [C]. It is predicted that by 2040, the number of cases will have risen from 1.850 million now to 3.093 million in 2040 [D].
This huge burden remains a challenge despite the expert consensus view that CRC is one of the most preventable cancers [E]. Five decades ago, the WHO developed criteria for a public health approach to screening providing that evidence and health burden justified it and it was feasible [F]. Three decades ago, the crucial evidence emerged. Guaiac-based fecal occult blood tests (gFOBT) had been proven to reduce mortality through early detection of neoplastic lesions [G].
With this evidence that early diagnosis of colorectal cancer (CRC) has a major impact on mortality (and incidence) it has now been shown that its use in practice is effective and feasible. Many countries now use fecal occult blood tests (FOBT) for CRC screening in the form of either guaiac FOBT (gFOBT) or the newer, and now the international standard, fecal immunochemical tests for hemoglobin (FIT)[G]. In 2018, all but two of the 30 countries with an age-standardized CRC incidence rate (ASR, 0-85 years) of 30 per 100,000 or greater, have organized screening fully underway, while considering those with an incidence rate ≥25/100,000 an additional six were conducting pilot studies.
Studies have shown that FIT reduces CRC mortality as well as incidence through detection and removal of early stage cancers or pre-cancerous adenomas[H]. Higher sensitivity FIT are also useful in detection of advanced precursor lesions which when removed is known ro reduce incidence of CRC.
The development of quantitative FIT has improved detection of colorectal neoplasia and is now the predominant FOBT screening technology used worldwide [G]. In screening populations, FIT has a sensitivity of between 74-90.5% for colorectal cancer (CRC) [I] and 25-56% for advanced adenoma.
FIT have proved superior to gFOBT in various ways: increased participation rates (single stool sample and simpler collection technique), automated analyzer for objective and consistent measurement, increased sensitivity for cancer and adenomas, no significant drug or dietary interference and an objective adjustable endpoint that can be tailored to available colonoscopy capacity and expectations of test performance[J]. FIT eventually replaced gFOBT and became a game-changer for two-step screening [G].
Quantitation of stool hemoglobin (Hb) concentration has provided flexibility for those managing screening programs to choose the criterion value (the threshold or “cut-off” Hb concentration at which a test is reported as positive) that triggers diagnostic verification by colonoscopy [H]. This in turns facilitates planning of the health services capacity to manage the resultant colonoscopy workload [H].
In the 1980s it became apparent that FIT provided an alternative to the gFOBT. By 2012, the Expert Working Group on FIT (of the WEO Colorectal Cancer Screening Committee) identified 47 different FIT systems on the market [K]. Most were simply qualitative, using a set and inflexible positivity threshold (cut-off) for triggering colonoscopic follow-up.
Since then, several key issues have driven how FIT are used in CRC screening. The first is the recognition that population-based organised screening (PBOS) is the public health strategy being applied in most countries [G}. This has been to ensure high quality in all steps of the multistep screening pathway from invitation to detection and then impact on CRC mortality and/or incidence. Quantitative FIT provided the means to deliver sometimes-strained colonoscopy resources to those who needed it most.
The second was that a fixed, inflexible positivity threshold did not necessarily suit the public health goals of a PBOS program. The positivity threshold determines test accuracy – hence lesion detection and specificity – and colonoscopy workload, and ultimately cost-effectiveness. Programs wanted to be able to adjust the positivity threshold to suit test accuracy and workload consistent with program goals [K]. This required highly accurate quantitation of the faecal haemoglobin level (f-Hb) which enabled the program to choose a program-suitable positivity threshold.
The third was that due to the high volume of testing needed, automation of sample processing as well as automated assay providing an objective measurable test read-out was essential [K].
It has become apparent that only a few of the 47 tests were able to meet these needs.
In this two-step screening strategy where a FIT is used to identify a subpopulation more likely to have neoplasia (see Figure), it has been identified [K] that there are three sequential phases in the screening pathway – pre-analytic, analytic and post-analytic – each of which has its own quality considerations.
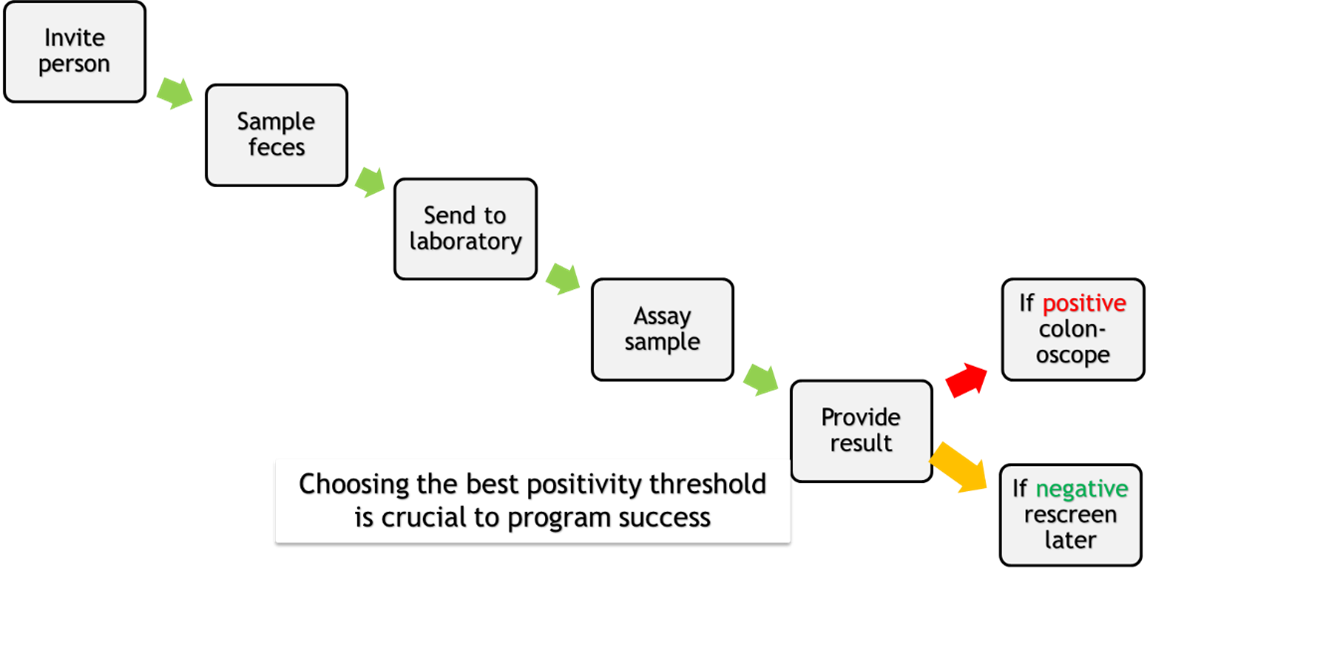
Figure: FIT-relevant steps in the multistep process of screening for CRC.
Pre-analytic considerations [K, L]
Clinical trials have shown that FIT-dependent pre-analytic considerations include:
・The nature and ease of the sampling method
・The adequacy of instructions
・The stability of Hb during transit [M]
Specific items needing attention include:
・Collection device usage - High quality instructions must be provided to ensure correct sampling and to avoid contamination with urine. Lack of dexterity in older people should be considered.
・Collection device design – The probe for collection of feces and the filter through which it is inserted must be carefully designed to control as far as possible the amount of feces collected. The buffer volume in the device should be tightly controlled to give a consistent fecal dilution and buffer composition must ensure stability of Hb between sample collection and assay at the laboratory (noting the potential for high temperatures during transit).
・Return to the laboratory – must be facilitated in a timely manner.
Each of these specific items might need some customisation in collaboration with manufacturers for the population being screened.
Without return of a correctly completed stable sample for assay, lesions will not be detected. Participation is the crucial program quality measure for this phase.
Analytical considerations
Once the sample arrives at the laboratory, all aspects of the analytical process are crucial to ensure accuracy at the positivity threshold ultimately considered appropriate for the program. Determining what this is might require sizeable population pilot programs to ensure that applicable program goals are met.
Recent WEO EWG recommendations on new non-invasive test evaluation, see [H] for further detail, stipulate:
“the analytical performance characteristics of the test must be formally documented according to relevant standards, such as those of the international Clinical and Laboratory Standards Institute or the Quality System Requirements of the USA.”
It is important that a FIT meets the analytical performance characteristics required for its use. Manufacturers and laboratories must comply with regulatory bodies to ensure that they provide accurate, reliable and reproducible results under a range of conditions applicable to sampling, handling, transport, measurement and reporting of results [see H].
A reliable result depends on:
・Precision and Trueness of the measure.
・Its Linearity (expressed as the measurement range).
・The Detection limits (of quantitation (LoQ), of detection (LoD), and of blank (LoB). The chosen positivity threshold must be above these values to ensure accuracy.
・Consistency of measurement across instrument sites and across time at a site.
Regulatory approval requires that such are demonstrated and ongoing accreditation of laboratories should be in place.
Thus, the ideal analytic process in a laboratory for a quantitative FIT will provide [K]:
・Automated reliable reading of participant identity from the sample device.
・Automated sampling of the buffer using a high capacity, automated analytical assay system.
・Consistent results under conditions of widespread use.
・Quality control processes that are reviewed regularly including external quality assurance processes that meet applicable standards.
・A policy for triggering the steps needed for repeating sample collection if the sample provided is unsatisfactory.
・A result in a manner applicable to the program (who is to be notified and the nature of the report – quantitative and/or qualitative).
While some of these are laboratory-dependent, manufacturers are encouraged to facilitate achievement of these ideals.
To meet regulatory frameworks, a manufacturer is required to demonstrate that their test meets applicable analytical requirements. To be used in practise, they are also required to provide clinical data on test utility (clinical accuracy) in a specified study population and based on a nominated f-Hb positivity threshold. This will guide screening providers in choice of test.
Post-analytic considerations
While post-analytic considerations are the responsibility of those responsible for conducting the screening screening program, manufacturers can facilitate selection of a test in several ways.
The first is to provide information on how to convert the sample buffer hemoglobin concentration to a concentration in the stool (f-Hb) by correcting for the amount of stool usually collected into the sample device buffer and the dilution effect of the buffer. This would facilitate comparing measurement results between different FIT assay systems [K]. Unfortunately, there are no international reference preparations that provide a standard for direct comparison of different FIT tests but this conversion is the first step for harmonization of different assay systems. Such preparations would also facilitate external quality control programs for laboratories.
Manufacturers are therefore encouraged to indicate quantitative results as mcg Hb/g faeces.
The second is that reporting quantified results allows the end-user to choose the f-Hb concentration that serves as the positivity threshold and so determines if the result is positive or negative and requires follow-up colonoscopy. While reporting quantitative results, to use a threshold not approved by a regulatory body might be an off-label use of a test in a few jurisdictions. In practice, there is a wide range of positivity thresholds in use around the world, from 10-120 mcg Hb/g [data in preparation for publication] and most jurisdictions do not have this limitation.
Being able to choose a positivity threshold allows a screening provider to choose test accuracy that suits a program goal. It also allows programs to determine how best to efficiently manage the colonoscopy workload in situations such as the SARS-CoV-2 pandemic.
There is now emerging evidence that there is advantage in being able to adjust the positivity threshold according to age and gender as these are determinants of f-Hb in these demographic subpopulations. Using a single f-Hb threshold might not be equitable across all subpopulations of age and gender.
How the positivity threshold is set for a program is ultimately for the program providers to decide and is usually identified by initial feasibility and pilot studies.
Manufacturers provide the crucial means for achieving program goals in FIT-based screening. How their test is configured is relevant, not just for the analytical laboratory aspects but also for key pre-analytic and post-analytic elements of a screening program.
A Begg SJ, Vos T, Barker B, et al. Burden of disease and injury in Australia in the new millennium: measuring health loss from diseases, injuries and risk factors. Med J Aust 2008;188:36-40.
B Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. . Lyon, France: International Agency for Research on Cancer, 2018. https://gco.iarc.fr/today
C Ferlay J, Colombet M, F B. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9 [Internet]. Lyon, France: International Agency for Research on Cancer, 2018. http://ci5.iarc.fr/CI5plus/Pages/references.aspx]
D http://gco.iarc.fr/tomorrow/home
E Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: World Cancer Research Fund / American Institute for Cancer Research, 2007; Lauby-Secretan B, Vilahur N, Bianchini F, et al. The IARC Perspective on Colorectal Cancer Screening. N Engl J Med 2018;378:1734-1740.
F Wilson JMG, Jungner G. Principles and practice of screening for disease. WHO Public Health Papers. Volume No. 34, 1968.
G Young GP, Rabeneck L, Winawer SJ. The Global Paradigm Shift in Screening for Colorectal Cancer. Gastroenterology. 2019;156: 843-851 e842.
H Bresalier RS et al. , Members of the World Endoscopy Colorectal Cancer Screening New Test Evaluation Expert Working Group. An efficient strategy for evaluating new non-invasive screening tests for colorectal cancer: the guiding principles. Gut, 2023; 72:1904-1918. Epub ahead of print doi:10.1136/gutjnl-2023-329701
I Lee, J.K., et al., Accuracy of Fecal Immunochemical Tests for Colorectal Cancer. Annals of Internal Medicine, 2014; 16: p. 171-181
J Young GP, Cole SR. Which fecal occult blood test is best to screen for colorectal cancer? Nat Clin Pract Gastroenterol Hepatol 2009;6:140-141.]
K Benton SC et al. Faecal immunochemical tests for haemoglobin: Analytical challenges and potential solutions. Clin Chim Acta 2021;517:60]
L Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP, Kuipers EJ, Seaman HE. Advances in Fecal Occult Blood Tests: The FIT Revolution. Digestive Diseases and Sciences. 2015; 60: 609-622.
M Symonds EL, Osborne JM, Cole SR, Bampton PA, Fraser RJL, Young GP. Factors affecting faecal immunochemical test positivity rate: effect of demographic, pathological, behavioural and environmental variables. Journal of Medical Screening, 2015 Dec;22(4):187-93.